
| # | Vendor code | Title |
|---|---|---|
| NG-0001 | NanoGraft bone composite 1 gr. | |
| NG-0003 | NanoGraft bone composite 3 gr. |
Synthetic bone composite Nano Graft (NanoGraft) is made on the basis of calcium hydroxyapatite and tricalcium phosphate with the possible addition of biologically active components of mineral, phyto- and organic origin (bischophyte, collagen and its derivatives, chitosan, eugenol, nano-, macro- and microelements etc.).
Dentistry, maxillofacial surgery, Orthopedics, traumatology, reconstructive surgery.
Glass vials and/or ampoules and disposable blister packaging. Materials for implantation.
NanoGraft- is a composite of crystalline calcium-deficient apatite (GAP) (at least 65%) and amorphous calcium phosphate (at least 35%) with a porous structure (45- 60% porosity). HAP is represented by non-stoichiometric calcium-deficient hydroxyapatite with free OH groups (Ca/P ratio – from 1.64 to 1.68). Able to influence the processes of hemostasis by activating fibrinogen with the formation of insoluble fibrin, which forms a three-dimensional mesh around the GAP/TCF-EXIMA granules. In this way, a physiological three-dimensional frame is formed for the adhesion of platelets and progenitor cells, which contribute to the release of growth factors and the formation of full-fledged bone tissue at the implantation site. In addition, calcium ions of amorphous calcium phosphate participate in the activation of the blood coagulation system due to their influence on the conversion of blood coagulation factors VIIa, IXa, X, as well as the formation of cross-linked insoluble fibrin.
In general, the use of HAP/TCF-EXIMA as an osteoplastic material allows the formation of a dense framework of fibrin fibers, which provides a basis for cell adhesion and bone tissue formation with an additional hemostatic effect.
ToV "Garantia Med", Ukraine, 69034, Zaporizhzhia, str. Jabotinsky, 40
The basic base is produced:
Bone defects of various genesis, contour bone plastic, osteoporosis, periodontitis, periodontitis, creation of conditions for implantation.
Any disorder or disease that may cause an unacceptable increase in postoperative risk. Infection in the implantation area.
After contact with blood, the divalent iron of hemoglobin interacts with free OH groups of calcium-deficient apatite with the formation of iron hydroxide (II) - Fe(OH)2, which has a greenish color. In the presence of oxygen (open air), iron (II) hydroxide is oxidized to iron (III) hydroxide – Fe(OH)3. Both compounds are physiological products of iron oxidation in the body and are non-toxic.
Before use and for ease of use, it can be moistened with isotonic solution, antibiotic solution or platelet-enriched plasma of the immediate patient. The material must be completely isolated from the external environment by covering fabrics. It is recommended to use the NanoPrime patch (membrane).
Not detected.
None.
When preserving the integrity of the glass package - five years from the date of sterilization. The date of sterilization is indicated on the package.
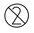 |
Reuse is PROHIBITED |
 |
ISPLEARN TO - this symbol is accompanied by a date consisting of four digits of the year, two digits of the month, and, if necessary, two digits of the day. The date must be written next to the symbol, or below it, or to the right of it. |
 |
DATE OF MANUFACTURE — for active implantable medical devices, the symbol will be combined with the date marked with four digits of the year and two digits of the month. For active products, the symbol must be followed by the year. The date must be marked after or below the symbol |
 |
STERILITY - only for medical devices that are fully sterilized. It can be applied with clarification of sterilization methods |
 |
WARNING! READ THE ACCOMPANYING DOCUMENTS —also can be synonymous with the symbol "Attention, see the instructions for use" |
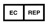 |
AUTHORIZED REPRESENTATIVE IN THE EUROPEAN UNION — this symbol must be accompanied by the name and address of the authorized representative in the European Union |
 |
GETTING TO KNOW THE INSTRUCTIONS FOR USE |
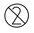 |
Reuse is PROHIBITED |